Enthalpy Change of Combustion
Standard enthalpy of combustion ΔH_C is the enthalpy change when 1 mole of a substance burns combines vigorously with oxygen under standard. My results show that hexanes 12278.

Specific Heat Enthalpy Molar Mass Combustion Equations Hope They Are Helpful To Anyone In Need Chemistr Chemistry Lessons Teaching Chemistry Chemistry Help
Standard Enthalpy of Combustion.

. Combustion reactions are exothermic so the value for the enthalpy change Delta H is always negative. When a substance undergoes combustion it releases energy. Enthalpy Change Definition Enthalpy change is the heat change accompanying a chemical reaction at constant volume or constant pressure.
For example standard enthalpy changes of combustion start with 1 mole of the substance you are burning. Hydrogen has the formula H 2 this represents 1 mole of hydrogen. In this case the equations need you to burn 6 moles of carbon and 3 moles of.
All the combustion reactions from the alcohols will be exothermic. Enthalpy Change of Combustion of Hydrogen. I have completed a preliminary test in my module developing fuels as an activity 12.
The enthalpy change of combustion will always have a negative value of course because burning always releases heat. Enthalpy is a thermodynamic quantity that is equivalent to the total heat content of a system as measured by a systems pressure and. I have experimented using the two fuels methanol and hexane.
Enthalpy of combustion equations will often. To determine the enthalpy change of combustion of Ethanol C 2 H 5 OH Propanol C 3 H 7 OH and Butanol C 4 H 9 OH. Question Ethanol C 2 H 5 OH was placed in a spirit burner and used to heat.
Standard Enthalpy of Combustion. Can enthalpy of combustion be positive. ΔH q n Δ H.
The standard enthalpy of change of a reaction can be calculated using Hess law and the standard enthalpy changes of combustion in cases where both sides of the equation can be burned in. Enthalpy changes and standard states. Chemicals Ethanol C 2 H 5 OH.
Hess law states that the change in enthalpy of the reaction is the sum of the changes in enthalpy of both parts. The enthalpy of combustion is calculated by dividing by the mole of alcohol consumed during complete combustion assuming there is no energy lost to the surrounding. Combustion is always exothermic the enthalpy change for the.
We also measure enthalpy change in J mol-1 or kJ mol-1. In this case the combustion of one mole of carbon has H. Show the equation that represents the standard enthalpy change of combustion of hydrogen.
Enthalpy change H is the amount of heat energy transferred during a chemical reaction at constant pressure. This chemistry video tutorial explains how to calculate the enthalpy change of a reaction using the enthalpy of formations found in the appendix section of y. The enthalpy change ΔH for a reaction represents the amount of heat energy released when the reactants are changed into products during a.
The enthalpy change or heat change when 1 mole of a substance is completely burnt in excess of oxygen or air is called enthalpy of Combustion or. H 2 g ½O 2. The enthalpy change tells the.
Exothermic is a process that releases heat in an exothermic reaction the enthalpy of the reacting system decreases is. Enthalpy of combustion It is the change in enthalpy accomplished when one mole of an element is heated in the presence of excess oxygen under standard conditions.

Enthalpy Heat Combustion Experiment 4 Energy Level Heat Exothermic Reaction

Enthalpy Of Combustion Easy Science Learn Physics Chemical Energy Thermodynamics
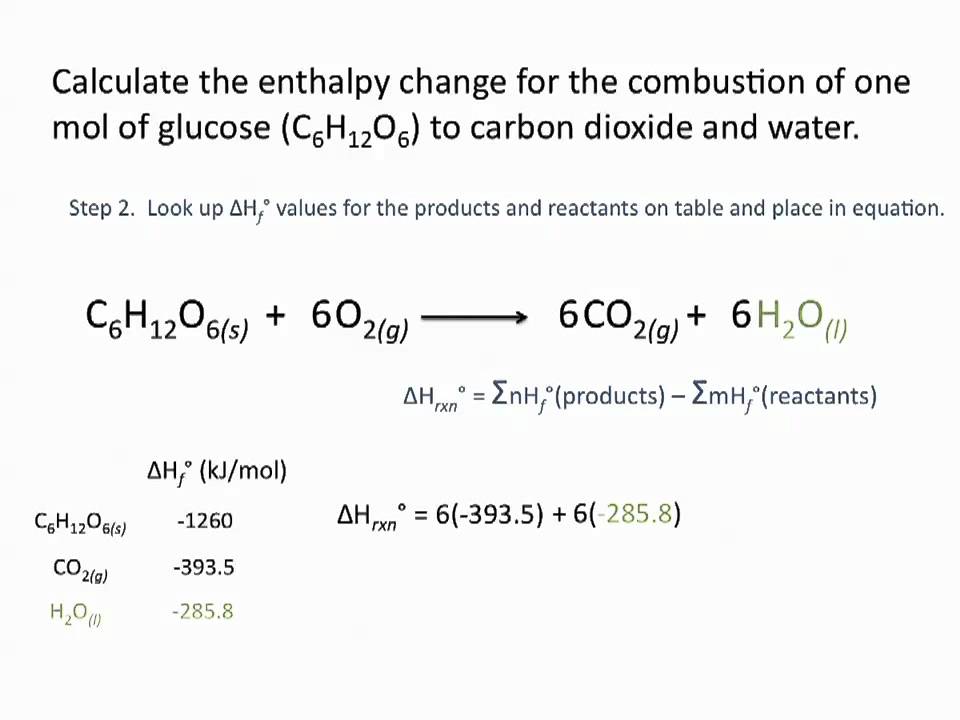
Enthalpies Of Formation Chemsitry Tutorial Science Chemistry Chemistry Tutorial

Total 2 Average 5 X2f 5 What Is The Heat Of Combustion What Is The Definition Of Enthalpy Chemistry Lessons Chemistry Classroom How To Study Physics
0 Response to "Enthalpy Change of Combustion"
Post a Comment